Maxwell boltzmann distribution pogil answer key – Welcome to the realm of the Maxwell-Boltzmann distribution POGIL answer key, where we embark on an enlightening journey into the fascinating world of particle behavior. This key unlocks a treasure trove of insights into the fundamental principles governing the motion and distribution of particles within a gas, providing a crucial foundation for understanding numerous phenomena in physics and chemistry.
Delving deeper into the Maxwell-Boltzmann distribution, we will explore its mathematical formulation, uncovering the intricate relationship between particle velocity and energy. We will also delve into its key features, examining how temperature influences the distribution and how it can be harnessed to predict the behavior of particles in various scenarios.
Maxwell-Boltzmann Distribution
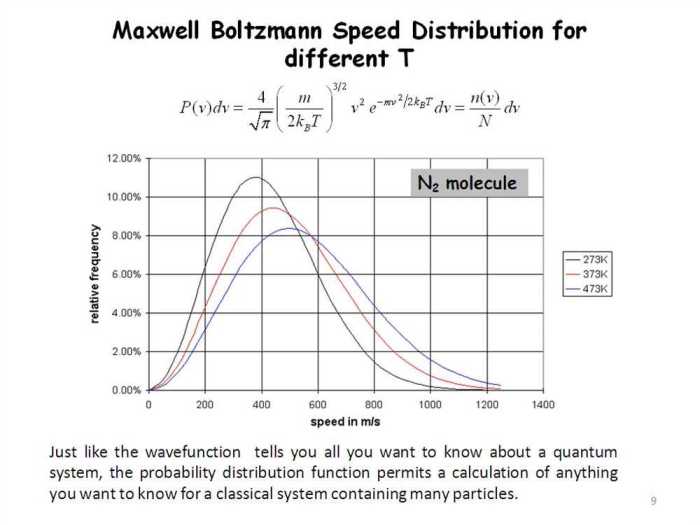
The Maxwell-Boltzmann distribution is a statistical distribution that describes the distribution of speeds in a gas. It is named after James Clerk Maxwell and Ludwig Boltzmann, who independently developed the distribution in the 19th century.
The Maxwell-Boltzmann distribution is given by the following equation:
$$f(v) = \sqrt\fracm2\pi kT e^-\fracmv^22kT$$
where:
- $f(v)$ is the fraction of molecules with speed $v$
- $m$ is the mass of a molecule
- $k$ is the Boltzmann constant
- $T$ is the temperature in Kelvin
Key Features of Maxwell-Boltzmann Distribution
The Maxwell-Boltzmann distribution has several key features:
- It is a Gaussian distribution, meaning that the distribution is symmetric around the mean.
- The mean of the distribution is the most probable speed of the molecules in the gas.
- The width of the distribution is proportional to the square root of the temperature.
- The distribution is only valid for gases that are in thermal equilibrium.
The Maxwell-Boltzmann distribution can be used to predict the behavior of particles in a gas. For example, it can be used to calculate the average speed of the molecules in a gas, or to predict the fraction of molecules that have a given speed.
Applications of Maxwell-Boltzmann Distribution in Physics and Chemistry
The Maxwell-Boltzmann distribution has a wide range of applications in physics and chemistry.
In physics, the Maxwell-Boltzmann distribution is used to:
- Calculate the average speed of the molecules in a gas
- Predict the fraction of molecules that have a given speed
- Study the behavior of gases in thermal equilibrium
In chemistry, the Maxwell-Boltzmann distribution is used to:
- Calculate the rate of chemical reactions
- Study the diffusion of gases
- Study the behavior of gases in non-equilibrium conditions
Limitations of Maxwell-Boltzmann Distribution, Maxwell boltzmann distribution pogil answer key
The Maxwell-Boltzmann distribution is a powerful tool for studying the behavior of gases. However, it has several limitations:
- It is only valid for gases that are in thermal equilibrium.
- It does not take into account the interactions between molecules.
- It does not apply to particles that are moving at relativistic speeds.
Despite these limitations, the Maxwell-Boltzmann distribution is a valuable tool for understanding the behavior of gases.
Top FAQs: Maxwell Boltzmann Distribution Pogil Answer Key
What is the Maxwell-Boltzmann distribution?
The Maxwell-Boltzmann distribution is a statistical distribution that describes the probability of finding a particle with a given velocity in a gas. It is based on the assumption that particles in a gas are in constant random motion and that their velocities are distributed according to a Gaussian distribution.
What are the key features of the Maxwell-Boltzmann distribution?
The Maxwell-Boltzmann distribution has several key features, including:
- It is a Gaussian distribution, meaning that the probability of finding a particle with a given velocity is highest at the mean velocity and decreases exponentially as the velocity deviates from the mean.
- The mean velocity of particles in a gas increases with increasing temperature.
- The distribution can be used to predict the behavior of particles in a gas, such as their average velocity, mean free path, and diffusion coefficient.
What are the applications of the Maxwell-Boltzmann distribution?
The Maxwell-Boltzmann distribution has numerous applications in physics and chemistry, including:
- Predicting the behavior of gases in various processes, such as effusion, diffusion, and thermal conductivity.
- Determining the temperature of a gas.
- Modeling the behavior of particles in plasmas and other non-ideal gases.
What are the limitations of the Maxwell-Boltzmann distribution?
The Maxwell-Boltzmann distribution has some limitations, including:
- It assumes that particles in a gas are non-interacting, which is not always the case in real gases.
- It does not account for quantum effects, which can become significant at very low temperatures.

