Conjugate acid base pairs worksheet with answers – Embark on a journey into the realm of conjugate acid-base pairs with our comprehensive worksheet and answer key. Dive deep into the fascinating world of acid dissociation and base dissociation, unraveling the intricacies of these fundamental chemical reactions.
This meticulously crafted worksheet provides an array of acid-base pairs, challenging you to identify and write their corresponding conjugate pairs. Test your understanding and solidify your grasp of this essential chemistry concept.
Conjugate Acid-Base Pairs Worksheet with Answers: Conjugate Acid Base Pairs Worksheet With Answers
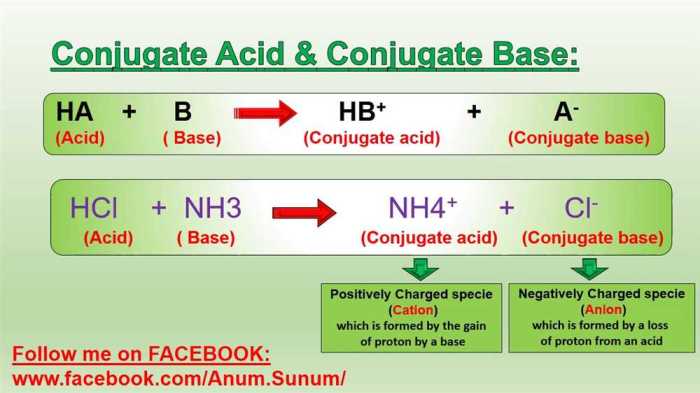
Introduction
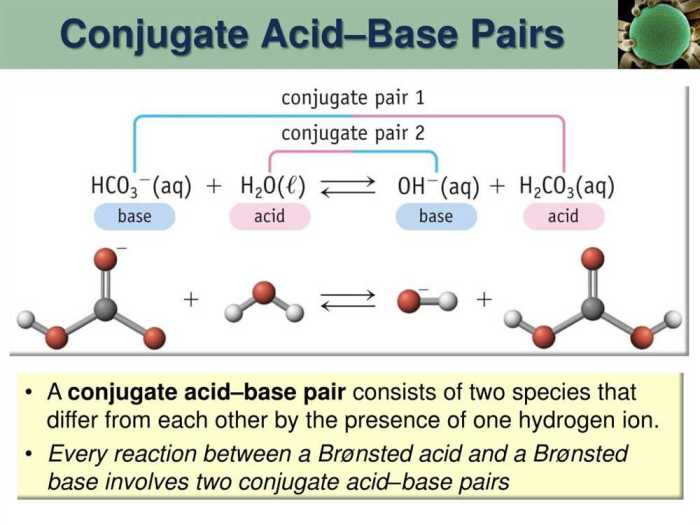
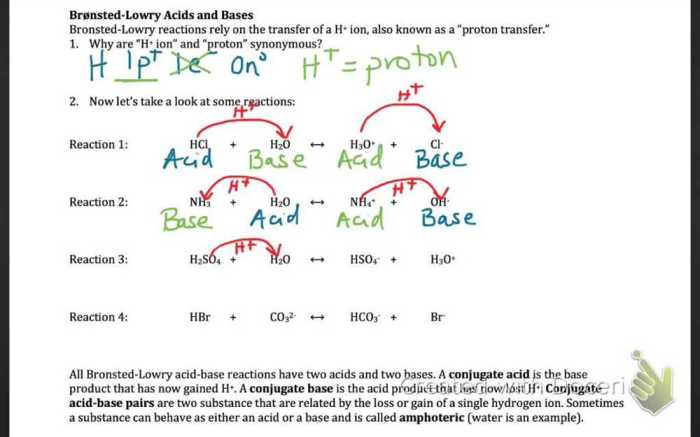
In chemistry, conjugate acid-base pairs are two species that differ by a single proton (H+ ion). When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid.
Worksheet Content
Acid-Base Pairs:
- HCl and Cl-
- H2SO4 and HSO4-
- NH3 and NH4+
- H2O and OH-
- CH3COOH and CH3COO-
Identify the Conjugate Acid-Base Pairs:
- Identify the conjugate acid of H2PO4-
- Identify the conjugate base of HSO4+
- Write the conjugate acid-base pair for HNO3
Answer Key
| Acid | Conjugate Base |
|---|---|
| HCl | Cl- |
| H2SO4 | HSO4- |
| NH3 | NH4+ |
| H2O | OH- |
| CH3COOH | CH3COO- |
| H2PO4- | H3PO4 |
| HSO4+ | H2SO4 |
| HNO3 | NO3- |
Additional Resources, Conjugate acid base pairs worksheet with answers
- Conjugate Acid-Base Pairs (Khan Academy)
- Conjugate Acid-Base Pairs Explained (YouTube)
- Acid-Base Solutions Interactive Simulation (PhET)
FAQs
What is the definition of a conjugate acid-base pair?
A conjugate acid-base pair consists of two species that differ by the presence of a proton (H+ ion). The acid donates a proton, forming its conjugate base, while the base accepts a proton, forming its conjugate acid.
How do I identify conjugate acid-base pairs?
To identify conjugate acid-base pairs, look for species with the same molecular formula but different charges. The acid will have a positive charge, while its conjugate base will have a negative charge.